Scientific/Technical Advisory
Provides support and scientific guidance in the design of studies and drafting of research protocols, ensuring the highest scientific and technical standards.
Offers scientific and regulatory advice to harmonise scientific and legal requirements, facilitating the efficient and timely authorisation of diagnostic and therapeutic tools, as well as medical devices when necessary.
Clinical Operations Development
To support independent clinical research, the Platform has a multidisciplinary team capable of carrying out the following activities at both national and international levels:
- Study design and methodology
- Start-up procedures and regulatory affairs
- Study coordination and monitoring
- Data management and statistical analysis
- Pharmacovigilance
- Scientific writing
Scope of Action
In general terms, and always within the field of independent clinical research, SCReN focuses its activities on conducting multicentre projects with high impact on the National Health System:
- Clinical trials involving drugs or medical devices (phases I–IV)
- Studies involving drugs or devices using non-trial designs
- Clinical trials with non-pharmacological interventions (e.g., surgical procedures)
- Observational studies with or without drugs
Reach
SCReN in Spain
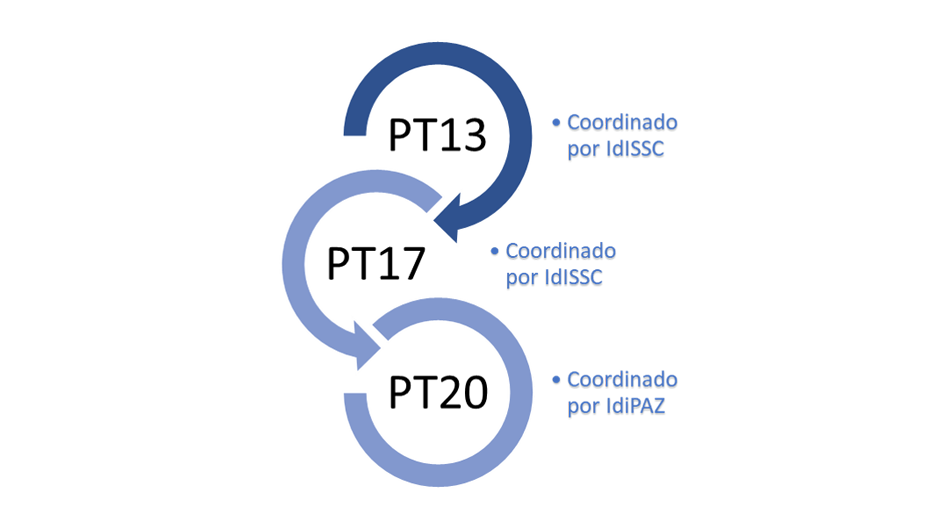
SCReN was created within the framework of the Strategic Action in Health (Acción Estratégica en Salud – AES) through the 2013 call, with the aim of:
- Providing high-level scientific, technical, and technological support to R&D&I projects in Health Sciences and Technologies, particularly integrated excellence projects from accredited Health Research Institutes (IIS). It also promotes cross-cutting initiatives within its area of activity.
- Boosting Spanish participation in international programmes and platforms.
- Promoting innovation in health technologies as a tool to contribute to the sustainability of the National Health System (NHS). Its development continued with the AES 2017 plan and is currently supported by the AES 2020 call.
SCReN is funded by the Instituto de Salud Carlos III (ISCIII) and co-funded by the European Regional Development Fund (ERDF).
SCReN in Europe
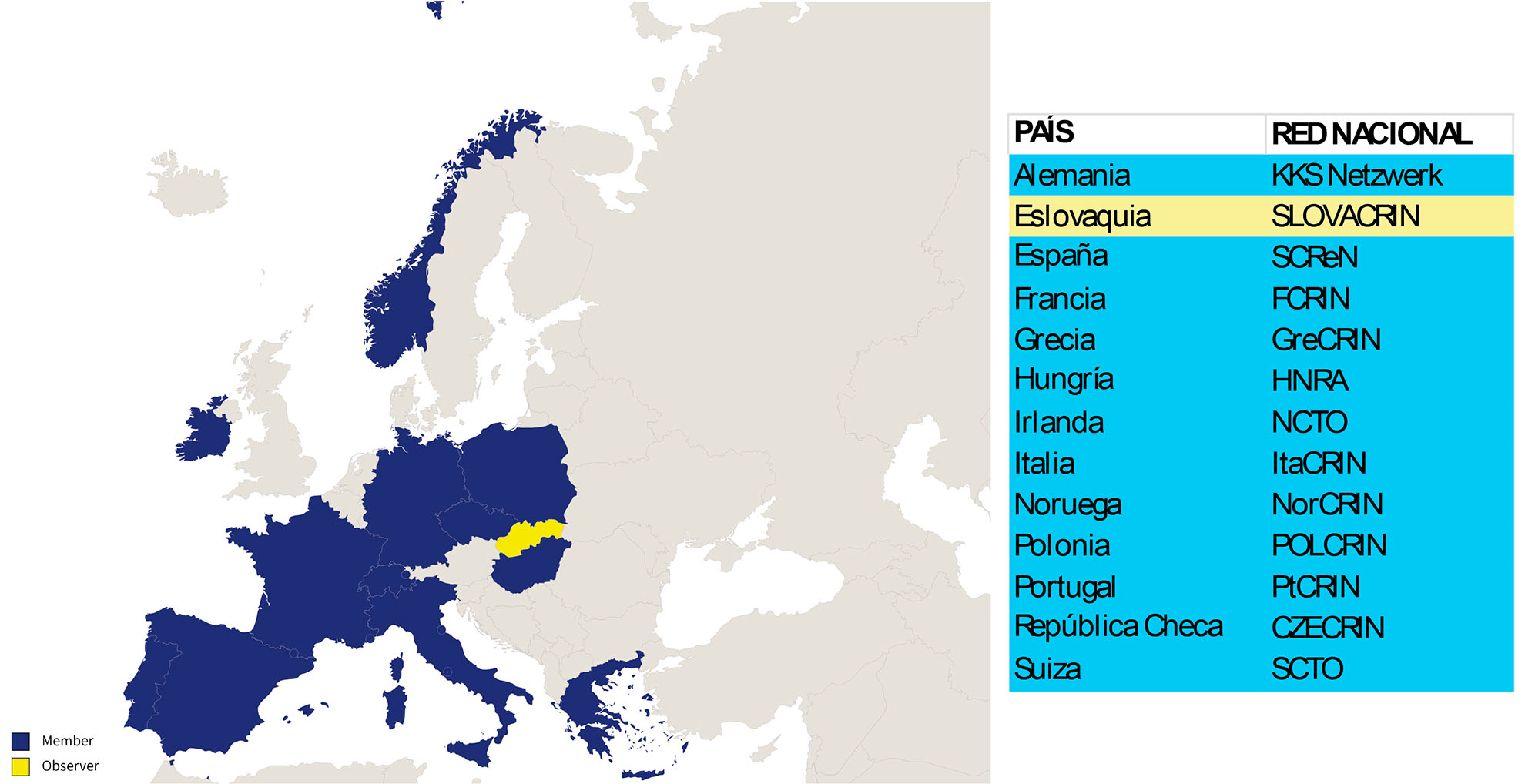
At the European level, SCReN is part of ECRIN (European Clinical Research Infrastructure Network), a non-profit organisation dedicated to supporting and managing independent multinational clinical trials. ECRIN connects national clinical research networks, providing expert advice and ensuring compliance with current European Union legislation, with the backing of its member states. Currently, 13 of the 28 EU countries are part of ECRIN, with the number expected to increase. Among these, 12 are full members and 1 holds observer status.
Global Situation
Since its inception, the Platform has supported both national and international studies. It currently manages 220 projects, 30 of which are part of ECRIN. The graph below shows the number of new projects incorporated each year:
| Year | Projects |
|---|---|
| 2013 | 19 |
| 2014 | 12 |
| 2015 | 15 |
| 2016 | 28 |
| 2017 | 31 |
| 2018 | 14 |
| 2019 | 18 |
| 2020 | 25 |
| 2021 | 41 |
| 2022 | 14 |